Old Fashioned Day Stone Mountain Nc

Limestone: A night grey, fine-grained specimen of the Middle Mississippian Greenbrier Limestone from Randolph County, eastern West Virginia. Specimen shown is about two inches (v centimeters) across.
What is Limestone?
Limestone is a sedimentary rock composed primarily of calcite, a calcium carbonate mineral with a chemic composition of CaCO3. It usually forms in clear, calm, warm, shallow marine waters.
Limestone is usually a biological sedimentary rock, forming from the accumulation of shell, coral, algal, fecal, and other organic debris. It can also class by chemical sedimentary processes, such as the atmospheric precipitation of calcium carbonate from lake or body of water water.
Table of Contents

A Limestone-Forming Environment: An underwater view of a coral reef arrangement from the Kerama Islands in the E People's republic of china Sea southwest of Okinawa. Hither the entire seafloor is covered by a wide variety of corals which produce calcium carbonate skeletons. A United states of america Geological Survey image by Brusk Storlazzi.
Biological Limestones
Well-nigh limestones class in calm, clear, warm, shallow marine waters. That type of environment is where organisms capable of forming calcium carbonate shells and skeletons can thrive and easily extract the needed ingredients from ocean water.
When these animals die, their shell and skeletal debris accrue as a sediment that might be lithified into limestone. Their waste product products also contribute to the sediment mass.
Limestones formed from this blazon of sediment are biological sedimentary rocks. Their biological origin is often, but not always, revealed in the rock by the presence of fossils.
Sometimes evidence of a biological origin is destroyed by the action of currents, organisms, dissolution, or recrystallization.
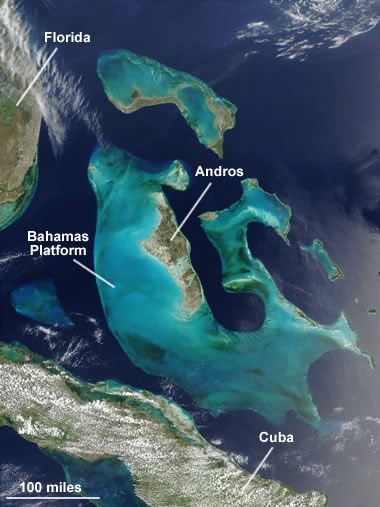
The Bahamas Platform: A NASA satellite image of the Bahamas Platform where agile limestone formation occurs today. The main platform is over 100 miles broad, and a corking thickness of calcium carbonate sediments accept accumulated there. In this image the dark blue areas are deep ocean waters. The shallow Bahama islands Platform appears every bit low-cal blue. Enlarge image.
Chemical Limestones
Some limestones form by direct precipitation of calcium carbonate from marine or fresh h2o. Limestones formed this way are chemical sedimentary rocks. They are thought to be less abundant than biological limestones.
Near biological limestones contain pregnant amounts of directly precipitated calcium carbonate. Later the biological grains have accumulated and are buried, water that is saturated with dissolved materials moves slowly through the sediment mass. Calcium carbonate, precipitated straight from solution, forms equally a "cement" that binds the biological grains together.
"Cementation" is an important step in the transformation of a sediment into a rock. If the biological grains are not cemented together, a rock volition not be formed. The amount of precipitated calcium carbonate in a biological limestone can be as low as a few percent of the rock past volume, or information technology can exist higher than 50% of the rock by volume.
Limestone-Forming Environments
Many limestone-forming environments are active on Earth today. Nigh of them are found in shallow parts of the body of water between 30 degrees northward breadth and thirty degrees south breadth.
Limestone is forming in the Caribbean area Body of water, Indian Sea, Western farsi Gulf, Gulf of Mexico, effectually Pacific Ocean islands, and inside the Indonesian archipelago.
One of these areas is the Bahamas Platform, located in the Atlantic Ocean about 100 miles southeast of southern Florida (see satellite image). There, abundant corals, shellfish, algae, and other organisms produce vast amounts of calcium carbonate skeletal droppings and fecal matter that completely blanket the platform. This is producing an extensive deposit of calcium carbonate sediment that has already converted to limestone at depth.
 | Limestone Stalactite A h2o drib clings to a stalactite. If it evaporates instead of falling, whatever dissolved calcium carbonate will add to the stalactite. National Park Service photo. |

Travertine: A view within the Cave of Balzarca in the Czech republic. Hither stalactites, stalagmites, and flowstone adorn the roof, floor, and walls of the cave. These rocks are a multifariousness of limestone known as travertine. Image copyright iStockphoto / JackF.
Evaporative (Cave) Limestones
Limestone tin besides form through evaporation. Stalactites, stalagmites, and other cave formations (often called "speleothems") are examples of limestone that formed through evaporation.
In a cavern, droplets of water seeping downwardly from above enter the cave through fractures or other pore spaces in the cave ceiling. In that location they might evaporate earlier falling to the cavern floor.
When the h2o evaporates, whatever calcium carbonate that was dissolved in the water will be deposited. Over fourth dimension, this evaporative process tin effect in an accumulation of icicle-shaped calcium carbonate on the cavern ceiling. These features are known as stalactites.
If droplets autumn to the floor and evaporate there, stalagmites could eventually grow upwards from the cave floor.
The limestone that makes up these cave formations is known as "travertine," a chemical sedimentary rock. A rock known as "tufa" is a limestone formed by evaporation at a hot spring or on the shoreline of a lake in an barren area.
Limerick of Limestone
Limestone is past definition a rock that contains at least l% calcium carbonate in the form of calcite by weight. All limestones contain at least a few percent other materials. These can exist minor particles of quartz, feldspar, or clay minerals delivered to the site by streams, currents and wave action. Particles of chert, pyrite, siderite, and other minerals tin form in the limestone by chemic processes.
The calcium carbonate content of limestone gives it a property that is ofttimes used in rock identification - it effervesces in contact with a cold solution of 5% muriatic acid. See our article about the "acid test" for identifying carbonate rocks and minerals.
Types of Limestone
There are many unlike types of limestone - each with its ain name. These names are often based upon how the rock formed, its appearance, its composition, or its physical properties. Here are some of the more than commonly encountered types of limestone.

Chalk: A fine-grained, light-colored limestone formed from the calcium carbonate skeletal remains of microscopic marine organisms.
Chalk
Chalk is the name of a limestone that forms from an accumulation of calcareous shell remains of microscopic marine organisms such as foraminifera. It tin can also form from the calcareous remains of some marine algae.
Chalk is a friable limestone with a very fine texture, and it is easily crushed or crumbled. It is unremarkably white or light grayness in color.
In the by pieces of natural chalk were used to write on blackboards. Today, most blackboard chalk is a man-made product. Some of it is made from natural chalk along with additives that improve its performance.

Coquina: This photograph shows the shell hash known as coquina. The stone shown hither is nigh two inches (five centimeters) across.
Coquina
Coquina is the name of a poorly cemented limestone composed almost exclusively of sand-size fragments of calcareous shell and/or coral debris. A small amount of calcareous cement ordinarily binds the grains together.
The sediments that form coquina accumulate on beaches where moving ridge action delivers an abundance of locally produced biological grains, while a significant amount of other material is non deposited. Coquina might be composed of clam, gastropod, brachiopod, trilobite, coral, ostracod or other invertebrate remains. See accompanying photograph or read an entire commodity about coquina hither.

Crystalline Limestone: A specimen of limestone that has been subjected to metamorphism. Some might call this fabric "crystalline limestone" - nevertheless, the proper name is marble. If you view this rock closely past middle, or better, with a hand lens, you will conspicuously run into cleavage faces of calcite intersecting at rhombic angles. The stone shown here is nigh four inches (10 centimeters) across.
Crystalline Limestone
When limestone is subjected to rut, pressure, and chemical activity, the calcite in the rock begins to transform. This is the beginning of the process known as metamorphism.
Starting at a microscopic scale, the calcium carbonate in the rock begins to crystallize or recrystallize into fine-grained calcite crystals. As the elapsing and intensity of metamorphism continues, the calcite crystals increase in size. When the calcite crystals are large enough to be visible to the middle, the rock can then be recognized equally marble - a metamorphic stone.
Marble is the proper name of the metamorphic rock that forms when limestone is subjected to the heat and pressure of metamorphism. It is composed of calcium carbonate (CaCOthree) and normally contains other minerals that might include clay minerals, micas, quartz, pyrite, fe oxide, and graphite.

Dolomitic Limestone: A view of the Kaibab Limestone at Walnut Canyon National Monument, Arizona. At this location, and many other locations, the Kaibab Limestone is fossiliferous and dolomitic. Photograph past the Usa Geological Survey.
Dolomitic Limestone
Dolomitic limestone is a rock composed mainly of calcite, but some of that calcite has been contradistinct to dolomite.
Dolomite is thought to course when the calcite (CaCOthree) in carbonate sediments or in limestone is modified by magnesium-rich groundwater. The available magnesium facilitates the conversion of calcite into dolomite (CaMg(COthree)2). This chemical change is known as "dolomitization."
Dolomitization can completely change a limestone into a dolomite, or it can partially alter the rock to form a "dolomitic limestone."

Fossiliferous Limestone: Ammonite fossils constitute in limestone quarry in Germany. Ammonite fossils are abundant in the area effectually Nuremberg and Stuttgart. Image copyright iStockphoto / hsvrs.
Fossiliferous Limestone
Fossiliferous limestone is a limestone that contains obvious and abundant fossils. They are ordinarily marine invertebrates such as brachiopods, crinoids, mollusks, gastropods, and coral. These are the normal trounce and skeletal fossils found in many types of limestone.
Fossiliferous limestone oft contains data about the environment of deposition, and where the organisms lived (or were deposited). Paleontologists can often examine the fossils and determine the geologic age of the stone.

Lithographic Limestone: In 1908, workers at NOAA's printing shop ink a slab of lithographic limestone that contains an image of a nautical chart. In 1900, NOAA produced approximately 100,000 lithographic prints using this method. A ingather from an image in the NOAA archive.
Lithographic Limestone
Lithographic limestone is a dense rock with a very fine and very uniform grain size. It occurs in sparse beds which separate easily to form a very smoothen surface.
In the late 1700s, a printing process known every bit lithography (named afterward the stones used) was developed to reproduce images by drawing them on the stone with an oil-based ink, and then using that stone to press multiple copies of the image.
Lithographic printing developed into an art class that produced many of the finest maps, navigational charts, posters, and bookplates of the 18th and 19th century. It was used by NOAA and the Usa military to produce millions of maps and navigational charts.
Printing with big stones weighing hundreds of pounds to over one ton was cumbersome work. Eventually lithographic printing was done using high-speed presses in which the prototype was inked on metallic rollers and transferred onto sheets or rolls of newspaper equally they streamed through the printing.

Oolitic Limestone: A specimen of limestone composed almost entirely of oolites. This rock was collected from the Salem Limestone, Middle Mississippian, at an unrecorded / undisclosed site in southern Indiana. Photo by James St. John, displayed here nether a Creative Eatables attribution license.
Oolitic Limestone
Oolites (or ooliths) are small, sand-size clasts of calcium carbonate with a spherical to ovate shape. They form past the concentric accumulation of calcium carbonate layers around a nucleus that might be a sand grain, a shell fragment, a coral fragment, or a particle of fecal debris. They are thought to form by inorganic precipitation of material around a nucleus while the clast is transported in wave-agitated waters or rolling beyond sediment surfaces.
In some parts of the Bahama islands Platform, oolites are one of the most abundant clasts found in the sediment. In areas where currents from deep h2o ascend onto the platform, broad areas are covered by bully thicknesses of sediment that is virtually entirely oolitic.
Oolitic limestone is found in many parts of the world. Oolitic sediment is found in Corking Salt Lake, Utah. Some sedimentary rocks are composed about entirely of ooids and the calcium carbonate cement that binds them together.

Travertine used as flooring tile and wall panels in a modernistic home interior. Epitome copyright iStockphoto / Katarzyna Bialasiewicz.
Travertine
Travertine is a diversity of limestone that forms where geothermally heated element of group i water, supercharged with dissolved gases and minerals, emerges at the surface. At that place, calcium carbonate and other minerals precipitate as the water degases and begins to evaporate.
Travertine tin can also form where these waters emerge into subsurface caverns. There, it tin can precipitate as cavern formations such every bit stalactites, stalagmites, and flowstone.
When pure, travertine is white, but information technology is often stained by the presence of other minerals to foam, tan, greenish, brownish, and other colors. Because the precipitation is rapid and forms as encrustations on younger materials, travertine is frequently a banded stone with numerous voids and cavities. It sometimes contains inclusions of organic and mineral debris from the cave or surface environment.
Travertine was mined and used as an architectural stone in aboriginal Egypt and ancient Rome. Today, Arab republic of egypt and Italy are famous sources of travertine that is exported throughout the globe. It is sawn or sheared into floor tiles, window sills, wall panels, stair treads, and other shapes, mainly for interior use. High-quality fabric can sometimes take a polish. The textile tin can exist recognized past its low hardness (three on the Mohs scale), banded appearance, and porous texture.

Tufa is a porous rock that forms from the atmospheric precipitation of calcium carbonate, frequently at a hot spring or along the shoreline of an element of group i lake where waters are saturated with calcium carbonate.

Tufa Towers are spectacular limestone features found in Mono Lake at Yosemite National Park in California. Image copyright iStockphoto / Andrew Soundarajan.
Tufa
Tufa is a porous limestone produced by precipitation of calcium carbonate from the waters of a hot leap or other body of surface water that has the ability to precipitate volumes of calcium carbonate. The pore space in tufa often results when plant fabric is trapped in precipitating calcium carbonate.
1 of the virtually famous locations where tufa is actively forming is at Mono Lake, Yosemite National Park. The most spectacular tufa features at the lake are known as "tufa towers". They grade by the interaction of freshwater springs and alkali metal lake water.
Evaporation around the edges of the lake helps produce the jagged shoreline tufa deposits and a lake that is nigh 2 1/two times as salty as the ocean and very alkali metal.
In spite of its gnarly appearance as a stone, tufa actually has numerous architectural uses. When institute in thick accumulations, tufa can be mined and sawn into blocks and sheets just like any other dimension stone. It produces a stone with a very rugged appearance.

Crushed Limestone: The Unsung Mineral Hero: Crushed stone is often looked upon as one of the lowliest of commodities; however, it is used for such a broad diversity of purposes in so many industries that it should be elevated to a position of distinction. It is the geologic commodity upon which almost everything is built. The Wordle word cloud above shows just a few of its many various uses.
"Unsung Mineral Hero" is a quote from the late Dewey Kirstein, Economic Geologist and i of the author'south early on supervisors. [1]
Uses of Limestone
Limestone is a stone with a multifariousness of uses. It could be the 1 rock that is used in more means than any other. Most limestone is made into crushed stone that is used in road base, railroad anchor, foundation stone, drainfields, concrete aggregate, and other construction uses. Information technology is fired in a kiln with crushed shale to brand cement.
Some varieties of limestone perform well in these uses because they are potent, dense rocks with few pore spaces. These properties enable them to stand up well to chafe and freeze-thaw. Although limestone does non perform besides in these uses equally some of the harder silicate rocks, it is much easier to mine and does not exert the same level of wear on mining equipment, crushers, screens, and the beds of the vehicles that transport it. In many parts of the globe, the harder silicate rocks are too far from construction sites to be used economically.

A Jewel of Crinoidal Limestone: This cabochon was cut from a piece of fossiliferous limestone that is rich in crinoid debris. Crinoids are organisms that take the morphology of stemmed plants just are actually animals. Rarely, crinoidal and other types of limestone have the ability to accept a brilliant polish and have interesting colors and patterns. These specimens can exist made into unusual and beautiful organic gems. This cabochon is about 39 millimeters square and was cut from textile plant in China.

Anti-Slip Aggregate: This image is a microscopic view of a polished surface of the Loyalhanna Limestone from Fayette County, Pennsylvania. The Loyalhanna is a Late Mississippian calcareous sandstone to arenaceous limestone, composed of siliceous sand grains embedded in and bound past a matrix of calcium carbonate. In outcrop, the Loyalhanna is cross-bedded with features that have caused geologists to argue if information technology is of marine bar or eolian dune origin. This view shows nearly one centimeter of rock betwixt opposing corners of the photo with sand grains measuring about 1/2 millimeter in diameter. As a construction material, the Loyalhanna is valued as an anti-sideslip aggregate (crushed stone). When it is used to make concrete paving, sand grains in amass particles exposed on a wet pavement surface provide traction for tires, giving the pavement an anti-sideslip quality.
Some additional but also important uses of limestone include:
Dimension Stone: Limestone is often cutting into blocks and slabs of specific dimensions for use in construction and in architecture. It is used for facing stone, floor tiles, stair treads, window sills, and many other purposes.Covering Granules: Crushed to a fine particle size, crushed limestone is used as a weather- and rut-resistant blanket on asphalt-impregnated shingles and roofing. It is also used as a elevation coat on congenital-upwards roofs.
Flux Stone: Crushed limestone is used in smelting and other metal refining processes. In the heat of smelting, limestone combines with impurities and can be removed from the process every bit slag.
Portland Cement: Limestone is heated in a kiln with shale, sand, and other materials and ground to a powder that will harden subsequently being mixed with water.
AgLime: Calcium carbonate is one of the most price-effective acid-neutralizing agents. When crushed to sand-size or smaller particles, limestone becomes an effective material for treating acidic soils. It has been widely used on fields and pocket-sized plots throughout the world for hundreds of years.
Lime: If calcium carbonate (CaC0three) is heated to high temperature in a kiln, the production will be a release of carbon dioxide gas (CO2) into the atmosphere and a residual product of calcium oxide (CaO). The calcium oxide is a powerful acid-neutralization agent. It is widely used every bit a soil treatment agent (faster interim than aglime) in agriculture and as an acid-neutralization amanuensis past the chemical industry.
Fauna Feed Filler: Chickens need calcium carbonate to produce stiff eggshells, so calcium carbonate is frequently offered to them equally a dietary supplement in the form of "craven grits." Information technology is too added to the feed of some dairy cattle who must supercede large amounts of calcium lost when the animal is milked.
Mine Safety Dust: Also known as "stone dust." Pulverized limestone is a white pulverisation that can be sprayed onto exposed coal surfaces in an underground mine. This brilliant white blanket improves illumination and reduces the amount of coal dust that becomes suspended in the air of the mine. This improves the air for breathing, and it as well reduces the explosion hazard produced by particles of combustible coal dust suspended in the air.
Limestone has many other uses. Powdered limestone is used as a filler in paper, pigment, rubber, and plastics. Crushed limestone is used equally a filter stone in on-site sewage disposal systems. Powdered limestone is also used as a sorbent (a substance that absorbs pollutants) at many coal-burning facilities.
Limestone is not found everywhere. It only occurs in areas underlain past sedimentary rocks. When limestone is needed in other areas, buyers sometimes pay v times the mine-site price of the stone in delivery charges so that limestone can exist used in their project or process.

Rock & Mineral Kits: Get a stone, mineral, or fossil kit to larn more than almost Earth materials. The best way to learn well-nigh rocks is to accept specimens available for testing and exam.
| Limestone Data |
| [one] Limestone: West Virginia'southward Unsung Mineral Hero: Dewey Kirstein; an article in Mountain Land Geology magazine, published past the West Virginia Geological and Economical Survey; pages 25-28, 1984. |
Find Other Topics on Geology.com:
 Rocks: Galleries of igneous, sedimentary and metamorphic rock photos with descriptions. |  Minerals: Information about ore minerals, gem materials and rock-forming minerals. |
 Volcanoes: Articles about volcanoes, volcanic hazards and eruptions by and present. |  Gemstones: Colorful images and articles about diamonds and colored stones. |
 General Geology: Articles nearly geysers, maars, deltas, rifts, common salt domes, water, and much more! |  Geology Shop: Hammers, field bags, hand lenses, maps, books, hardness picks, gold pans. |
 |  Diamonds: Acquire about the backdrop of diamond, its many uses, and diamond discoveries. |
0 Response to "Old Fashioned Day Stone Mountain Nc"
Post a Comment